Phisohex: Package Insert / Prescribing Info
Package insert / product label
Generic name: hexachlorophene
Dosage form: emulsion
Drug class: Antiseptic and germicides
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Phisohex Description
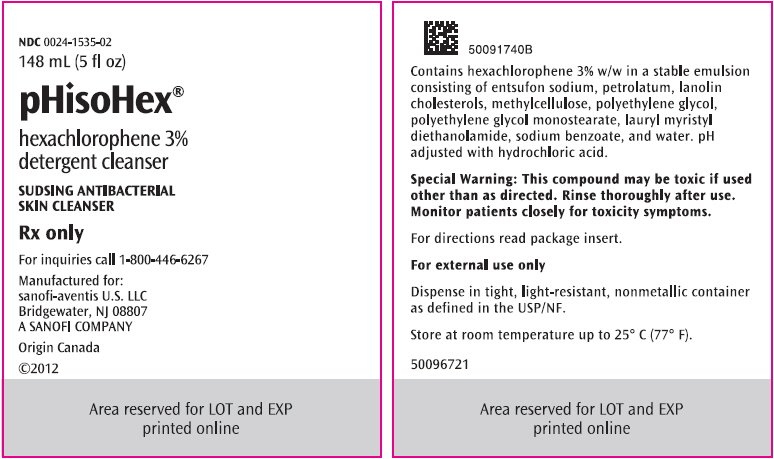
pHisoHex, brand of hexachlorophene detergent cleanser, is an antibacterial sudsing emulsion for topical administration. pHisoHex contains a colloidal dispersion of hexachlorophene 3% (w/w) in a stable emulsion consisting of entsufon sodium, petrolatum, lanolin cholesterols, methylcellulose, polyethylene glycol, polyethylene glycol monostearate, lauryl myristyl diethanolamide, sodium benzoate, and water. pH is adjusted with hydrochloric acid. Entsufon sodium is a synthetic detergent.
Chemically, hexachlorophene is Phenol, 2,2'-methylenebis[3,4,6-trichloro-; and has the following structural formula:
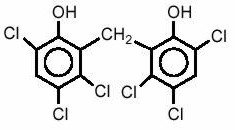
Phisohex - Clinical Pharmacology
pHisoHex is a bacteriostatic cleansing agent. It cleanses the skin thoroughly and has bacteriostatic action against staphylococci and other gram-positive bacteria. Cumulative antibacterial action develops with repeated use. Cleansing with alcohol or soaps containing alcohol removes the antibacterial residue.
Detectable blood levels of hexachlorophene following absorption through intact skin have been found in subjects who regularly scrubbed with hexachlorophene emulsion 3%. (See WARNINGS for additional information.)
pHisoHex has the same slight acidity as normal skin (pH value 5.0 to 6.0).
Indications and Usage for Phisohex
pHisoHex is indicated for use as a surgical scrub and a bacteriostatic skin cleanser. It may also be used to control an outbreak of gram-positive infection where other infection control procedures have been unsuccessful. Use only as long as necessary for infection control (see PRECAUTIONS: Information for Patients).
Contraindications
pHisoHex must not be used on burned or denuded skin.
pHisoHex must not be used for bathing infants (see WARNINGS). Infants may absorb the active compound in pHisoHex more readily than older children and adults. Such absorption has been associated with central nervous system effects such as convulsions.
It must not be used as an occlusive dressing, wetpack, or lotion. It must not be used routinely for prophylactic total body bathing.
It must not be used as a vaginal pack or tampon, or on any mucous membranes.
pHisoHex must not be used on persons with sensitivity to any of its components. It must not be used on persons who have demonstrated primary light sensitivity to halogenated phenol derivatives because of the possibility of cross-sensitivity to hexachlorophene.
Warnings
RINSE THOROUGHLY AFTER EACH USE. Patients should be closely monitored and use should be immediately discontinued at the first sign of any of the symptoms described below.
Rapid absorption of hexachlorophene may occur with resultant toxic blood levels when preparations containing hexachlorophene are applied to skin lesions such as ichthyosis congenita, the dermatitis of Letterer-Siwe's syndrome, or other generalized dermatological conditions. Application to burns has also produced neurotoxicity and death.
pHisoHex SHOULD BE DISCONTINUED PROMPTLY IF SIGNS OR SYMPTOMS OF CEREBRAL IRRITABILITY OCCUR.
Infants, especially premature infants or those with dermatoses, are particularly susceptible to hexachlorophene absorption. Systemic toxicity may be manifested by signs of stimulation (irritation) of the central nervous system, sometimes with convulsions.
Infants have developed dermatitis, irritability, generalized clonic muscular contractions and decerebrate rigidity following application of a 6 percent hexachlorophene powder. Examination of brainstems of those infants revealed vacuolization like that which can be produced in newborn experimental animals following repeated topical application of 3 percent hexachlorophene. Moreover, a study of histologic sections of premature infants who died of unrelated causes has shown a positive correlation between hexachlorophene baths and lesions in white matter of brains.
Precautions
General
Avoid accidental contact of pHisoHex with the eyes.
If contact occurs, promptly rinse thoroughly with water. To assist in the detection of ocular irritation, applications to the head and periorbital skin areas should be performed only in responsive patients with unanesthetized eyes.
RINSE THOROUGHLY AFTER USE, especially from sensitive areas such as the scrotum and perineum.
pHisoHex is intended for external use only. If swallowed, pHisoHex is harmful, especially to infants and children. pHisoHex should not be poured into measuring cups, medicine bottles, or similar containers since it may be mistaken for baby formula or other medications.
Information for Patients
The prescribing physician is requested to inform the patient about the following precautionary measures:
pHisoHex must not be used on burned or denuded skin. Application to burns has produced neurotoxicity and death.
pHisoHex must not be used for bathing infants (see WARNINGS). Infants may absorb the active compound in pHisoHex more readily than older children and adults. Such absorption has been associated with central nervous system effects such as convulsions.
pHisoHex must not be used as an occlusive dressing, wetpack, or lotion.
pHisoHex must not be used routinely for prophylactic total body bathing.
pHisoHex must not be used as a vaginal pack, or on any mucous membranes.
pHisoHex must not be used on persons with sensitivity to any of its components.
pHisoHex must not be used on persons who have demonstrated primary light sensitivity to halogenated phenol derivatives because of the possibility of cross-sensitivity to hexachlorophene.
pHisoHex should be kept out of the eyes. If contact occurs, the patient should rinse with cold water as soon as possible and contact a physician.
pHisoHex should not be used in sensitive areas such as the scrotum and perineum. If contact occurs, these areas should be rinsed thoroughly.
pHisoHex is for external use only.
If pHisoHex is inadvertently swallowed (see OVERDOSAGE), the patient should contact a physician or Poison Control Center as soon as possible.
pHisoHex should not be poured into measuring cups, medicine bottles, or similar containers since it may be mistaken for baby formula or other medications.
pHisoHex should be stopped and a physician should be contacted if irritation, sensitization, or allergic reaction occurs.
pHisoHex should be used in pregnant women or nursing mothers only if the potential benefit justifies the potential risk to the fetus or infant.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies in animals
Hexachlorophene was tested in one experiment in rats by oral administration; it had no carcinogenic effect.
Hexachlorophene was not mutagenic in Salmonella typhimurium and was negative in a dominant lethal assay in male mice. Cytogenetic tests with cultured human lymphocytes were also negative.
Embryotoxicity and Teratogenicity
Placental transfer of hexachlorophene has been demonstrated in rats.
Hexachlorophene is embryotoxic and produces some teratogenic effects.
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Hexachlorophene should be used during pregnancy only if the potential benefit justifies potential risk to the fetus.
Hexachlorophene has been shown to be teratogenic and embryotoxic in rats when given by mouth or instilled into the vagina in large doses.
Administration of 500 mg/kg diet or 20 to 30 mg/kg bw/day by gavage to rats caused some malformations (angulated ribs, cleft palate, micro- and anophthalmia) and reduction in litter size.
Placental transfer and excretion in milk of hexachlorophene has been demonstrated in rats.
In another study, doses of up to 50 mg/kg diet failed to produce any effects in 3 generations of rats. Hexachlorophene did not interfere with reproduction in hamsters.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hexachlorophene, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.
Pediatric Use
pHisoHex must not be used for bathing infants (see WARNINGS). Infants may absorb the active compound in pHisoHex more readily than older children and adults. Such absorption has been associated with central nervous system effects such as convulsions. For premature infants: see WARNINGS.
Geriatric Use
Clinical studies of pHisoHex did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, use in elderly patients should be cautious, reflecting the greater frequency of dermatological disease, peripheral circulatory disease, and decreased propensity for wound healing in this group. In addition, use in elderly patients should take into account any decreased hepatic, renal, or cardiac function, as well as any concomitant disease or other drug therapy.
Adverse Reactions/Side Effects
Adverse reactions to pHisoHex may include dermatitis and photosensitivity. Sensitivity to hexachlorophene is rare; however, persons who have developed photoallergy to similar compounds also may become sensitive to hexachlorophene.
In persons with highly sensitive skin the use of pHisoHex may at times produce a reaction characterized by redness and/or mild scaling or dryness, especially when it is combined with such mechanical factors as excessive rubbing or exposure to heat or cold.
Overdosage
The accidental ingestion of pHisoHex in amounts from 1 oz to 4 oz has caused anorexia, vomiting, abdominal cramps, diarrhea, dehydration, convulsions, hypotension, and shock, and in several reported instances, fatalities.
If patients are seen early, the stomach should be evacuated by emesis or gastric lavage. Olive oil or vegetable oil (60 mL or 2 fl oz) may then be given to delay absorption of hexachlorophene, followed by a saline cathartic to hasten removal. Treatment is symptomatic and supportive; intravenous fluids (5 percent dextrose in physiologic saline solution) may be given for dehydration. Any other electrolyte derangement should be corrected. If marked hypotension occurs, vasopressor therapy is indicated. Use of opiates may be considered if gastrointestinal symptoms (cramping, diarrhea) are severe. Scheduled medical or surgical procedures should be postponed until the patient's condition has been evaluated and stabilized.
Phisohex Dosage and Administration
Surgical Hand Scrub
- Wet hands and forearms with water. Apply approximately 5 mL of pHisoHex over the hands and rub into a copious lather by adding small amounts of water. Spread suds over hands and forearms and scrub well with a wet brush for 3 minutes. Pay particular attention to the nails and inter-digital spaces. A separate nail cleaner may be used. Rinse thoroughly under running water.
- Apply 5 mL of pHisoHex to hands again and scrub as above for another 3 minutes. Rinse thoroughly with running water and dry.
- For repeat surgical scrubs during the day, scrub thoroughly with the same amount of pHisoHex for 3 minutes only. Rinse thoroughly with water and dry.
Bacteriostatic Cleansing
Wet hands with water. Dispense approximately 5 mL of pHisoHex into the palm, work up a lather with water and apply to area to be cleansed.
Rinse thoroughly after each washing.
INFANT CARE
pHisoHex must not be used for bathing infants (see WARNINGS). Infants may absorb the active compound in pHisoHex more readily than older children and adults. Such absorption has been associated with central nervous system effects such as convulsions.
PREMATURE INFANTS
See WARNINGS.
Use of baby skin products containing alcohol may decrease the antibacterial action of pHisoHex.
How is Phisohex supplied
5 oz plastic squeeze bottle (NDC 0024-1535-02).
1 pint plastic squeeze bottle (NDC 0024-1535-06).
Store at room temperature up to 25° C (77° F)
Prolonged direct exposure of pHisoHex to strong light may cause brownish surface discoloration but does not affect its antibacterial or detergent properties. Shaking will disperse the color. If pHisoHex is spilled or splashed on porous surfaces, rinse off to avoid discoloration.
pHisoHex should not be dispensed from, or stored in, containers with ordinary metal parts. A special type of stainless steel must be used or undesirable discoloration of the product or oxidation of metal may occur.
Directions For Cleaning Dispensers
Before initial installation and use, run an antiseptic, such as an aqueous solution of benzalkonium chloride, NF. 1:500 to 1:750, or alcohol, through the working parts; rinse with sterile water. At weekly intervals thereafter, remove dispenser and pour off remainder of pHisoHex emulsion. Rinse empty dispenser with water. Run water through the working parts by operating the dispenser. Sanitize as described above. Rinse thoroughly with sterile water.
ANIMAL TOXICITY
The oral LD50 of hexachlorophene in male rats is 66 mg/kg bw, in females 56 mg/kg bw, and in weanling rats 120 mg/kg bw.
In suckling rats (10-days old), it is 9 mg/kg bw.
Manufactured for:
sanofi-aventis U.S. LLC, A SANOFI COMPANY
Bridgewater, NJ 08807
Revised January 2012
© 2012 sanofi-aventis U.S. LLC
PRINCIPAL DISPLAY PANEL - 148 mL Bottle Label
NDC 0024-1535-02
148 mL (5 fl oz)
pHisoHex®
hexachlorophene 3%
detergent cleanser
SUDSING ANTIBACTERIAL
SKIN CLEANSER
Rx only
For inquiries call 1-800-446-6267
Manufactured for:
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
A SANOFI COMPANY
Origin Canada
©2012
| PHISOHEX
hexachlorophene emulsion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Valeant Pharmaceuticals International, Inc. | 248902996 | MANUFACTURE(0024-1535) , ANALYSIS(0024-1535) , LABEL(0024-1535) , PACK(0024-1535) | |
More about pHisoHex (hexachlorophene topical)
- Check interactions
- Compare alternatives
- Reviews (2)
- Dosage information
- Drug class: antiseptic and germicides
